Click here to start your patients
on the path to just 2 DOSES A YEAR
- For US Healthcare Professionals Only
In the 5 pivotal trials for INVEGA SUSTENNA® in patients with schizophrenia, the most common adverse reactions (incidence ≥5% and occurring at least twice as often as placebo) were1:
No occurrences of adverse events reached this threshold in the 15-month, double-blind, placebo-controlled phase in patients with schizoaffective disorder.1
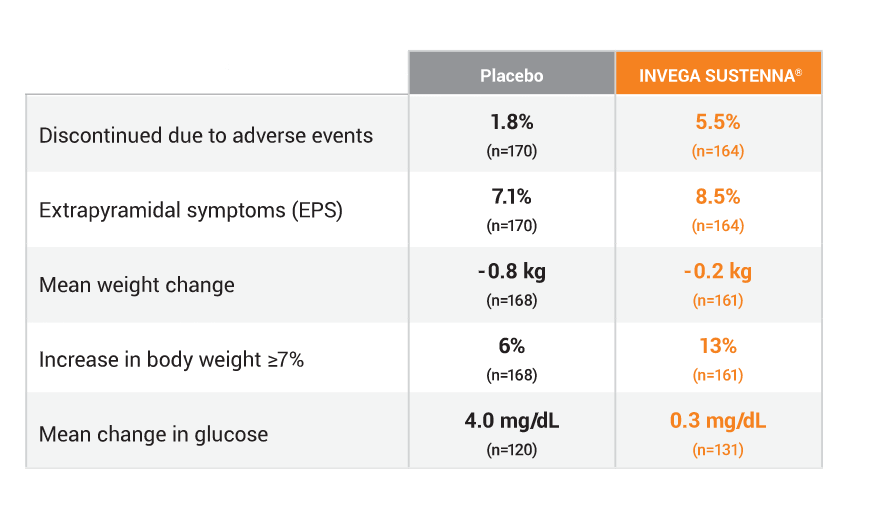
The following adverse reactions occurred more frequently (a ≥2% difference vs placebo) in the long-term study in patients with schizoaffective disorder1:
Indicated for the treatment of schizoaffective disorder.
The full constellation of symptoms and the relevant diagnostic criteria should be consulted and are available in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5 ®, or current version), where applicable.