Click here to start your patients
on the path to just 2 DOSES A YEAR
- For US Healthcare Professionals Only
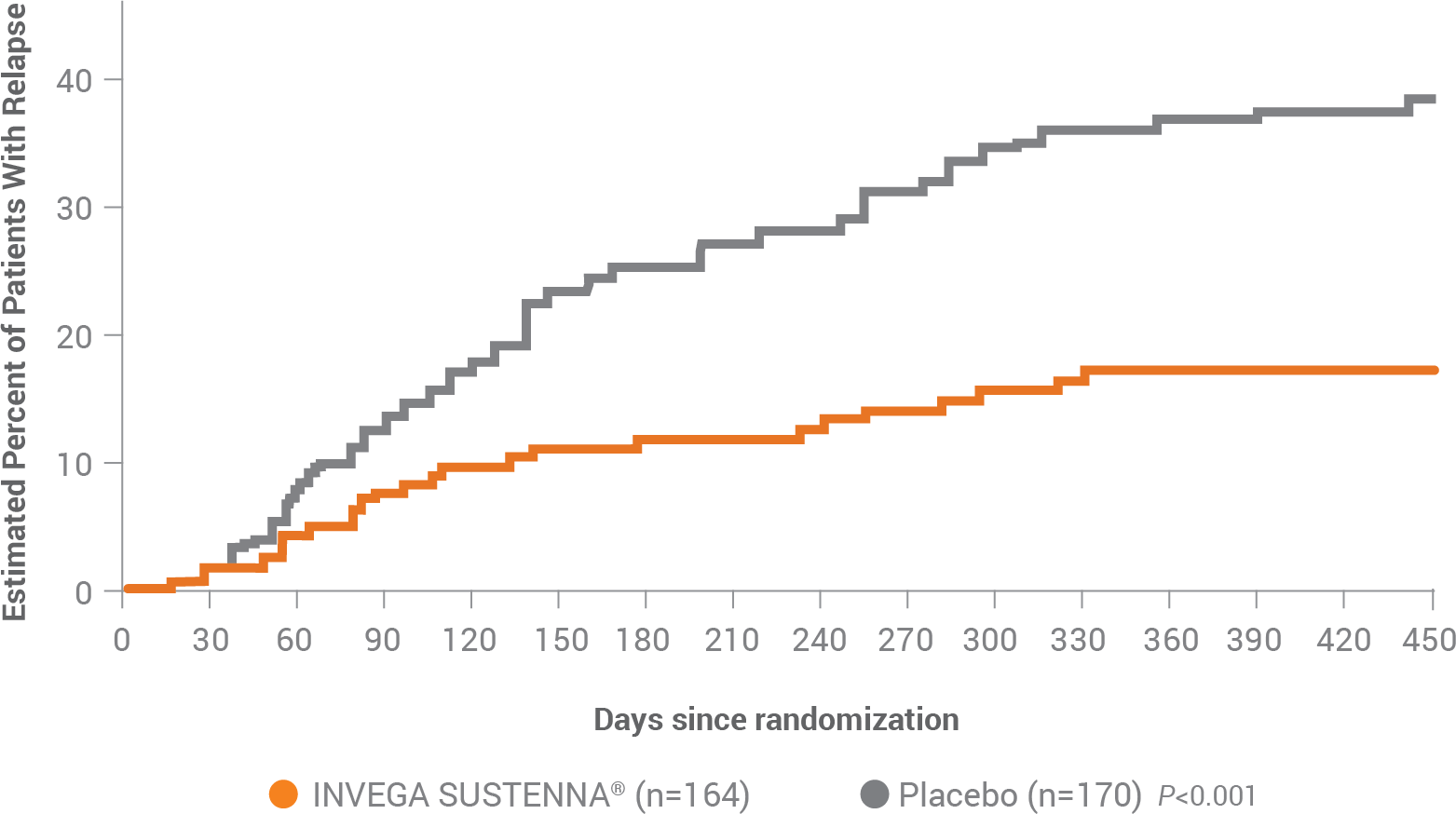
INVEGA SUSTENNA® helped more patients remain relapse-free vs placebo, both as1:
Monotherapy: 88% vs 67%
Adjunctive therapy*: 81% vs 66%
*Adjunct to antidepressants or mood stablizers.

Of the patients who relapsed on INVEGA SUSTENNA® (n=25) and placebo (n=57), the majority of patients experienced symptoms of both psychosis and mood at the time of their relapse. Eight of the 82 patients experienced a relapse without psychotic symptoms, and 16 of the 82 patients experienced a relapse without mood symptoms.1
Indicated for the treatment of schizoaffective disorder.
The full constellation of symptoms and the relevant diagnostic criteria should be consulted and are available in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5 ®, or current version), where applicable.
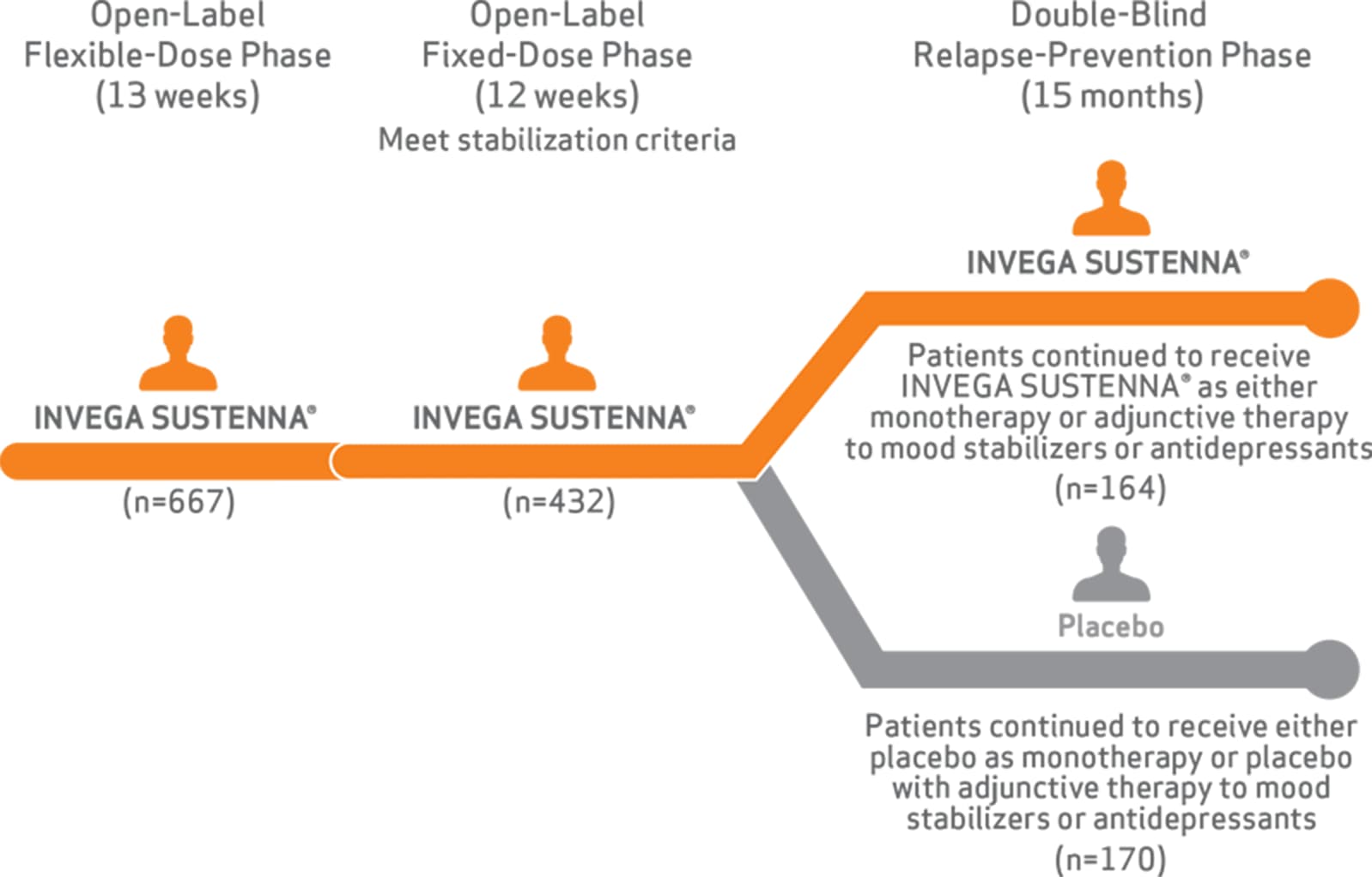
In patients experiencing an acute exacerbation of psychotic and manic/depressive mood symptoms
Double-blind baseline patient demographics (n=334)2:
Study Design
The primary endpoint was time to relapse. Adult patients with schizoaffective disorder were treated for 13 weeks during an open-label, flexible-dose lead-in phase with INVEGA SUSTENNA® as monotherapy and as an adjunct to mood stabilizers or antidepressants (78-mg, 117-mg, 156-mg, or 234-mg), followed by a 12-week, open-label, fixed-dose stabilization period. Patients who maintained stabilization criteria were then randomized to this same dose or to placebo in the 15-month, double-blind, maintenance phase.1,2
Indicated for the treatment of schizoaffective disorder.
The full constellation of symptoms and the relevant diagnostic criteria should be consulted and are available in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5 ®, or current version), where applicable.
PANSS=Positive and Negative Syndrome Scale; CGI-S-SCA=Clinical Global Impression for Severity of Schizoaffective Disorder.
†Defined as a score of ≥6 (if the score was ≤4 at randomization) of any of the individual PANSS items: delusions, conceptual disorganization, hallucinatory behavior, excitement, suspiciousness/persecution, hostility, uncooperativeness, or poor impulse control.
‡Defined as 2 consecutive assessments within 7 days showing ≥25% increase (if the score at randomization was >45) or ≥10-point increase (if the score at randomization was ≤45) in total PANSS score; a score of ≥5 (if the score was ≤3 at randomization) of any of the individual PANSS items: delusions, conceptual disorganization, hallucinatory behavior, excitement, suspiciousness/persecution, hostility, uncooperativeness, or poor impulse control; an increase of ≥2 points (if the score was 1 [not ill] to 3 [mildly ill] at randomization) or increase of ≥1 point (if the score was ≥4 [moderately ill or worse] at randomization) in CGI-S-SCA overall score.
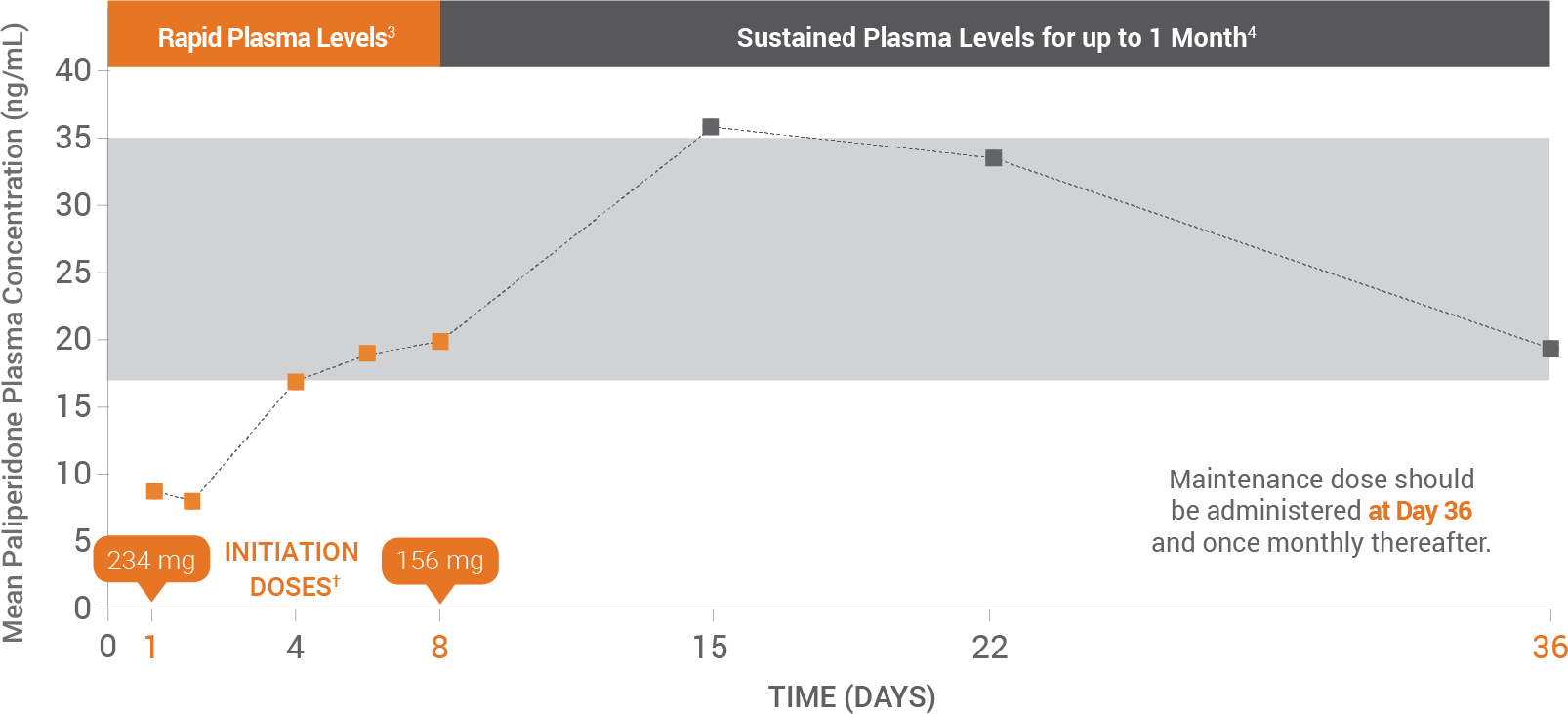
Due to the difference in median pharmacokinetic profiles between the 2 products, INVEGA SUSTENNA® and INVEGA® (paliperidone) oral, caution should be exercised when making a direct comparison of their pharmacokinetic properties.1
§After both initiation doses.
ll INVEGA SUSTENNA® initiation regimen allowed patients to stay in this exposure window of 6 mg to 12 mg extended-release oral paliperidone.
¶Initiation doses must be administered in the deltoid muscle.
#First maintenance dose should be administered on day 36 and once monthly thereafter.
Figure adapted with permission from Samtani MH et al. CNS Drugs. 2011;25(10):829-845.
Figure adapted with permission from Samtani MH et al. CNS Drugs. 2011;25(10):829-845.